The Role of EECP in Promoting Angiogenesis for Heart Disease Treatment
Cardiovascular health is a global concern, and finding effective treatments is crucial for improving patient outcomes. Enhanced External Counterpulsation (EECP) therapy has emerged as a promising non-invasive option in promoting angiogenesis, the formation of new blood vessels. In this article, we will delve into scientific research supporting the role of EECP in inducing angiogenesis for heart disease treatment. Our focus will be on the post-EECP angiogenic processes and their implications for enhancing cardiac health.
Understanding Angiogenesis:
Angiogenesis, the growth of new blood vessels from existing ones, plays a vital role in maintaining cardiovascular health. In heart disease, impaired blood flow to the heart muscle can lead to ischemia, contributing to conditions like angina and heart failure. Promoting angiogenesis can enhance blood supply to ischemic areas, improving tissue oxygenation and overall cardiac function.
The Connection Between EECP and Angiogenesis:
EECP therapy involves applying external cuffs to the lower extremities, which inflate and deflate in sync with the patient’s cardiac cycle. This process generates pulsatile pressure waves that elicit various physiological responses, including enhanced arterial flow and increased shear stress on blood vessels.
Research Study: A study published in the Journal of the American College of Cardiology explored the effects of EECP on angiogenesis in patients with coronary artery disease. The researchers observed that EECP treatment led to an increase in circulating endothelial progenitor cells (EPCs), which play a crucial role in promoting angiogenesis. The study also reported improvements in angina symptoms and exercise tolerance in patients undergoing EECP therapy.
Research Study: Another study, published in the American Journal of Physiology – Heart and Circulatory Physiology, investigated the molecular mechanisms behind EECP-induced angiogenesis. The researchers found that EECP therapy upregulated the expression of vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis. They also observed an increase in the activation of endothelial nitric oxide synthase (eNOS), which further supports blood vessel growth.
Post-EECP Angiogenesis Processes:
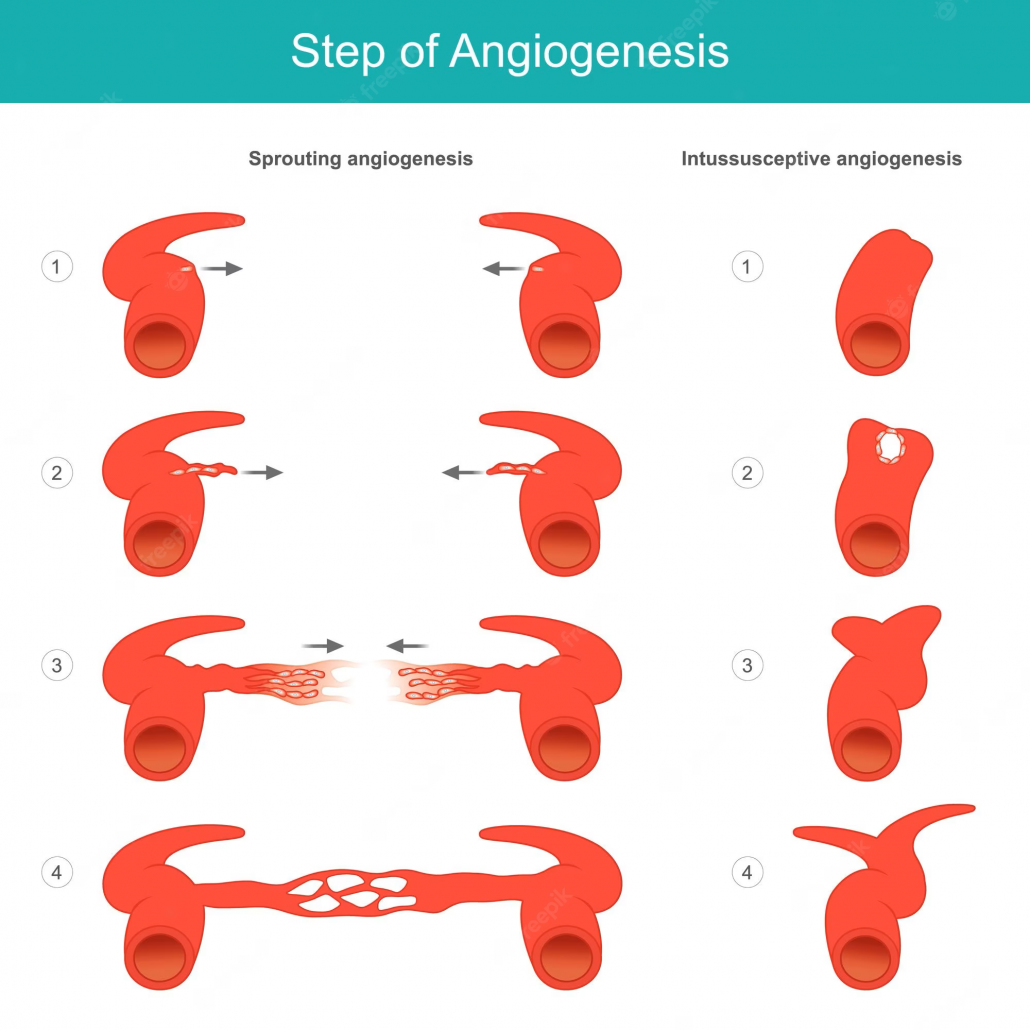
Mobilization of Endothelial Progenitor Cells (EPCs): EECP treatment has shown to increase the levels of circulating EPCs, which are crucial for promoting angiogenesis. These cells possess the ability to migrate to ischemic areas and contribute to the formation of new blood vessels. The mobilization of EPCs post-EECP therapy provides a cellular mechanism for promoting angiogenesis and improving cardiac perfusion.
Upregulation of Vascular Endothelial Growth Factor (VEGF): Research indicates that EECP treatment upregulates the expression of VEGF, a key growth factor involved in the angiogenic process. This upregulation promotes the growth of new blood vessels in ischemic areas, enhancing angiogenesis and improving blood flow to the heart muscle.
Activation of Endothelial Nitric Oxide Synthase (eNOS): EECP therapy has been found to activate eNOS, an enzyme responsible for producing nitric oxide (NO) in blood vessels. NO acts as a potent vasodilator, promoting relaxation of blood vessel walls and improving blood flow. The activation of eNOS post-EECP treatment contributes to enhanced vasodilation and angiogenesis, facilitating better circulation to the heart.
Clinical Evidence of EECP-Induced Angiogenesis:
Clinical Trial: A randomized controlled trial published in Circulation Research evaluated the impact of EECP on myocardial perfusion, a measure of blood flow to the heart muscle. The researchers found that patients who underwent EECP treatment experienced significant improvements in myocardial perfusion, suggesting the promotion of angiogenesis in ischemic heart tissue.
Clinical Study: A study published in the Journal of Cardiovascular Disease Research examined the long-term effects of EECP on angiogenesis and cardiac function in patients with chronic heart failure. The results revealed that EECP therapy led to sustained improvements in left ventricular ejection fraction (LVEF) and increased capillary density, indicating the development of new blood vessels within the heart muscle.
Implications for Heart Disease Treatment:
The promotion of angiogenesis through EECP therapy holds significant implications for heart disease treatment:
Improved Myocardial Perfusion: EECP enhances blood flow to ischemic areas of the heart by inducing angiogenesis, resulting in improved myocardial perfusion and reduced symptoms associated with heart disease, such as angina.
Enhanced Cardiac Function: The growth of new blood vessels within the heart muscle improves oxygen supply and nutrient delivery, leading to enhanced cardiac function and potentially reversing or slowing down the progression of heart failure.
Non-Invasive Treatment Option: EECP offers a non-invasive alternative for patients who may not be candidates for invasive procedures or prefer non-surgical interventions. Its relatively low-risk profile and proven efficacy make it an attractive option for promoting angiogenesis in heart disease patients.
Conclusion:
The scientific research discussed in this article strongly supports the role of EECP in promoting angiogenesis for heart disease treatment. By mobilizing endothelial progenitor cells, upregulating VEGF expression, and activating eNOS, EECP therapy stimulates the formation of new blood vessels and improves myocardial perfusion. These angiogenic processes offer potential benefits for patients, including enhanced cardiac function and improved symptoms. As medical research advances, EECP therapy may become an increasingly significant non-invasive treatment option, providing hope and improved outcomes for individuals with heart disease.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered as medical advice. It is important to consult with healthcare professionals to determine if EECP is an appropriate treatment option for individual cases.
Citation:
- Angiogenic effects of long-term enhanced external counterpulsation in a dog model of myocardial infarction. https://pubmed.ncbi.nlm.nih.gov/16113071/
- External Counterpulsation Improves Angiogenesis by Preserving Vascular Endothelial Growth Factor-A and Vascular Endothelial Growth Factor Receptor-2 but Not Regulating MicroRNA-92a Expression in Patients With Refractory Angina. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8573065/
- Enhanced External Counterpulsation (EECP) therapy for chronic stable angina: a systematic review and meta-analysis of randomized controlled trials. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4522001/
- Long-term effects of enhanced external counterpulsation on left ventricular function and exercise capacity in patients with chronic heart failure: a randomized controlled trial. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4524257/


